The 2026 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI) was held from January 8 to 10 in San Francisco, United States. At this conference, the results of two neoadjuvant studies of Henlius' innovative anti-PD-1 monoclonal antibody(mAb) HANSIZHUANG (serplulimab, trade name in Europe: Hetronifly®), in the field of MSS/pMMR locally advanced colon/rectal cancer were officially released in the form of posters, further highlighting the therapeutic potential of serplulimab in the field of gastrointestinal cancers. Serplulimab is the world's first and only anti-PD-1 mAb to have succeeded in a phase 3 perioperative gastric cancer registration study and is also the world's first anti-PD-1 mAb for first-line treatment of small cell lung cancer, achieving groundbreaking progress in both lung and gastric cancers.
In the field of gastrointestinal cancers, in addition to the approved esophageal squamous cell carcinoma (ESCC) indication, serplulimab is the first drug to receive Breakthrough Therapy Designation from the National Medical Products Administration (NMPA) Center for Drug Evaluation (CDE). As the world’s first perioperative regimen for gastric cancer to replace adjuvant chemotherapy with immunotherapy monotherapy, its New Drug Application (NDA) has been accepted by the CDE and granted Priority Review. Besides, an international, multicenter Phase 3 trial (ASTRUM-015) of serplulimab in combination with bevacizumab and chemotherapy as first-line therapy for metastatic colorectal cancer (mCRC) has completed patient enrollment, potentially addressing the clinical gap in first-line immunotherapy for non-MSI-H mCRC. At this conference, positive results of serplulimab from two investigator-initiated trials (IITs) for neoadjuvant therapy in locally advanced colon/rectal cancer were released. The data indicate that serplulimab in neoadjuvant therapy is expected to improve the chances of radical surgery and long-term survival rates for patients with locally advanced high-risk colon cancer, while also showing potential to provide a radiation-free treatment regimen for patients with locally advanced rectal cancer.
Serplulimab demonstrates unique advantages in treating various solid tumors via its differentiated mechanism. The drug not only induces stronger PD-1 internalization—reducing PD-1 receptor presence on T cells for rapid and potent immune activation [1]—but also minimizes PD-1-mediated recruitment of the co-stimulatory molecule CD28, thereby preserving CD28 signaling [2-4], enhancing downstream AKT activity [5], and promoting sustained T-cell activation. Focused on gastrointestinal cancers and lung cancer, serplulimab has been approved for the treatment of squamous non-small cell lung cancer (sqNSCLC), extensive-stage small cell lung cancer (ES-SCLC), ESCC, and non-squamous non-small cell lung cancer (nsqNSCLC). Up to date, it has been approved in over 40 countries and regions including China, the U.K., Germany, Singapore, and India, covering nearly half of the global population and accelerating global accessibility. The results of 4 pivotal trials of serplulimab were published in the Journal of the American Medical Association (JAMA), Nature Medicine, Cancer Cell and British Journal of Cancer, respectively. It has also received orphan drug designations granted by the US FDA, the European Commission, Swissmedic, Korea MFDS and Mexico COFEPRIS.
The results of the two studies released at this ASCO GI conference are as follows:
Title: Neoadjuvant short-course radiotherapy combined with CAPOX and PD-1 inhibitor for MSS/pMMR high-risk locally advanced colon cancer: A randomized, prospective, multicentre, phase II trial (TORCH-C).

Study design: This is a prospective, multicenter, randomized phase II trial (NCT05732493). A total of 120 LACC (T4/bulky N+M0,pMMR/MSS) patients will be randomized to either a chemotherapy group or a radiotherapy group. The chemotherapy group receives 4 cycles of CAPOX. The radiotherapy group receives SCRT(25Gy in 5 fraction)and 4 cycles of the PD-1 inhibitor (serplulimab) combined with CAPOX. The radiotherapy target volume includes only the primary colon tumor and enlarged lymph nodes, without elective nodal irradiation. After neoadjuvant therapy, patients will be evaluated for radical colon resection. The primary endpoint is pathological complete response (pCR) rate. The secondary endpoints include tumor downstaging, R0 resection, 3-year disease free survial (DFS), 3-year overall survival(OS), 3-year local recurrence-free survival and treatment-related toxicity
Results: As of August 31, 2025, 120 patients have been enrolled and randomized. Of thses, 79 patients have completed neoadjuvant treatment and surgery (43 in the chemotherapy group, 36 in the radiotherapy group). All 79 patients achieved radical resection, with R0 resection rates of 95.3% (41/43) in the chemotherapy group (95.3%, 41/43), and 97.2% (35/36) in the radiotherapy group. The pCR rate (TRG 0) was 11.6% (5/43) in chemotherapy group and 47.2% (17/36) in the radiotherapy group. The most common grade 3-4 adverse event (AE) among patients completing neoadjuvant treatment was thrombocytopenia (15.3%, 15/98), including 5 cases of grade 4 thrombocytopenia.
Conclusion: The combination of PD-1 inhibitor, SCRT, and chemotherapy shows promising efficacy and may significantly improve pCR rates in patients with MSS/pMMR highrisk LACC. This regimen may provide a new therapeutic option to achieve R0 resection and improve long-term survival.
Title: Neoadjuvant chemotherapy and serplulimab in MSS/pMMR locally advanced rectal cancer (FIRM): a phase II trial
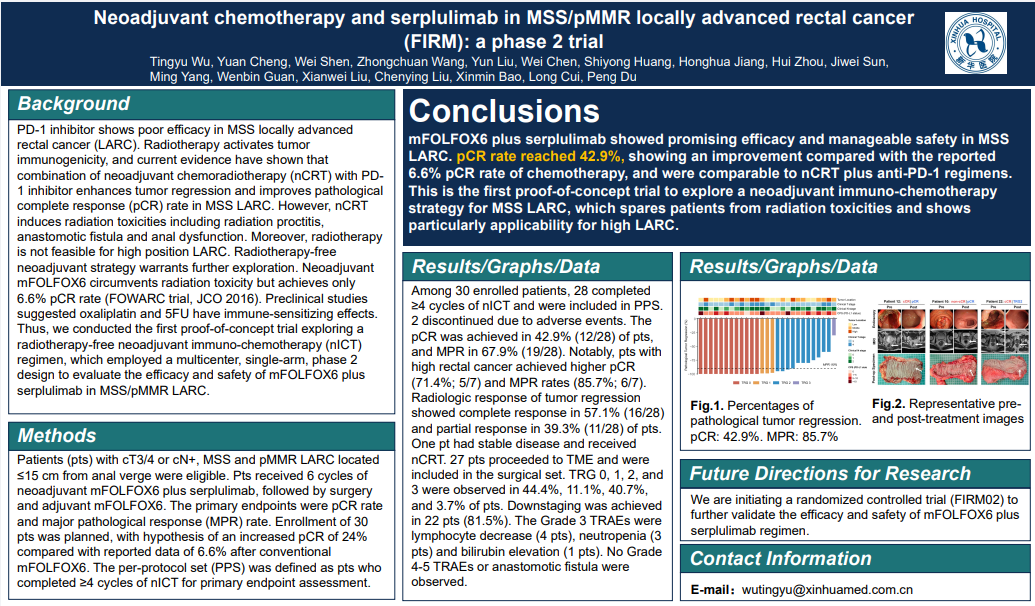
Study design: Patients (pts) with cT3/4 or cN+, MSS and pMMR LARC located ≤15 cm from anal verge were eligible. Pts received 6 cycles of neoadjuvant mFOLFOX6 and serplulimab, followed by surgery and adjuvant mFOLFOX6. The primary endpoints were pCR rate and major pathological response (MPR) rate.
Results: Among 30 enrolled patients, 28 completed ≥4 cycles of nICT and were included in PPS. 2 discontinued due to adverse events. The pCR was achieved in 42.9% (12/28) of pts, and MPR in 67.9% (19/28). For high LARC (>10 cm from anal verge, ineligible for nCRT), the pCR and MPR rates were 71.4% and 85.7%.
Conclusion: mFOLFOX6 plus serplulimab showed promising efficacy and manageable safety in MSS LARC. pCR rate unexpectedly reached 42.9%, showing an improvement compared with the reported 6.6% pCR rate of chemotherapy, and were comparable to nCRT plus anti-PD-1 regimens. This is the first proof of concept trial to explore a nICT strategy for MSS LARC, which spares pts from radiation toxicities and shows particularly applicability for high LARC.
References
[1] Issafras H, et al. Structural basis of HLX10 PD-1 receptor recognition, a promising anti-PD-1 antibody clinical candidate for cancer immunotherapy. PLoS One. 2021;16(12):e0257972.
[2] Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428-1433.
[3] Patsoukis N, et al. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun Biol. 2020;3(1):128.
[4] Fenwick C, et al. Tumor suppression of novel anti-PD-1 antibodies mediated through CD28 costimulatory pathway. J Exp Med. 2019;216(7):1525-1541.
[5] Primavera E, et al. Computer-Aided Identification of Kinase-Targeted Small Molecules for Cancer: A Review on AKT Protein. Pharmaceuticals (Basel). 2023;16(7):993.
